"GRIP Molecular is working with BARDA on a single-use, hand-held cartridge that is designed to enable lab-grade accuracy, multiplexed detection of SARS-CoV-2, influenza A and B, RSV, and other common pathogens using a nasal swab sample. Their proprietary biosensor technology is intended to provide results within five minutes and read-out on a standard mobile device."
Link to full article: https://www.medicalcountermeasures.gov/newsroom/2023/athometest/
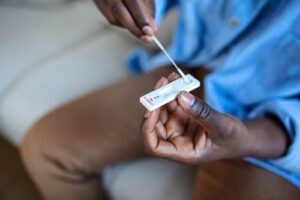
BARDA’s Division of Research, Innovation, and Ventures (DRIVe) and the Detection, Diagnostics, and Devices Infrastructure (DDDI) Division are partnering with multiple industry and academic partners to develop next-generation home-based, over-the-counter diagnostic technologies for the detection of SARS-CoV-2, influenza, and future pandemic preparedness.
At-home diagnostics can empower individuals with actionable information about their infection status so that they can self-isolate, which can help reduce community transmission, and seek medical care and treatment early in the course of any infection. Developing rapid and affordable home-based diagnostics, along with the technologies that support them, is essential to enabling widespread adoption of COVID-19 at-home testing.
Accelerating the capabilities of COVID-19 tests can propel these new diagnostic platforms for future use against other pathogens. For example, being able to detect and distinguish between viral respiratory illnesses that may present similarly – such as COVID-19, influenza, or respiratory syncytial virus (RSV) – is key to enabling early and improved infectious disease management.
BARDA is working with multiple partners to develop home-based, over-the-counter technologies to detect SARS-CoV-2 acute infection as part of DRIVe-DDDI’s “COVID-19 At-Home Diagnostics” program. The overall aim of this program is to make home-based infection detection more routine and accessible, by developing and validating more rapid, affordable, and highly accurate tests. Home-based diagnostics must also be easy and intuitive to use, with results that are simple and actionable to interpret. The initial portfolio of projects represents a wide range of technological platforms and underlying technologies, and each feature unique potential advantages, such as the ability to simultaneously detect multiple pathogens. Additionally, some technologies are designed to target detection of proteins specific to viruses like SARS-CoV-2, while others are targeting detection of its nucleic acids.
These partners all bring unique perspectives and innovative approaches to the program:
3EO Health is a diagnostic company located in Beverly, Massachusetts, that utilizes a modified isothermal molecular test to detect several SARS-CoV-2-specific genes from nasal swab samples. The proprietary swab clicks into a disposable cartridge which automates sample processing. The reusable base unit reports the test results through an LED display. U.S. Food and Drug Administration (FDA) Emergency Use Authorization (EUA) submission is expected in 2023.
Altratech, a biotechnology company based in Cork, Ireland, is developing SARS-CoV-2 detection tests leveraging an innovation in peptide nucleic acid-based (PNA) probes to increase sensitivity and specificity of unamplified molecular diagnostic tests from saliva samples. The test detects SARS-CoV-2 molecular targets using complementary metal–oxide–semiconductor (CMOS) fringe-field capacitive sensing.
Gladstone Institutes is an independent biomedical research institution in San Francisco, California. The Gladstone team, in collaboration with scientists at UC Berkeley, is working to develop a direct, non-amplified nucleic acid detection chemistry enabled by programmable Cas13a nuclease. The test will distinguish between SARS-CoV-2, influenza A and B, RSV A and B, and seasonal coronaviruses. The technology platform utilizes a disposable microfluidic cartridge and reusable base unit to perform molecular detection from nasal swabs.
GRIP Molecular Technologies is a diagnostics company based in Saint Paul, Minnesota. GRIP is developing a single-use, hand-held cartridge that is designed to enable lab-grade accuracy, multiplexed detection of SARS-CoV-2, influenza A and B, RSV, and other common pathogens using a nasal swab sample. Their proprietary biosensor technology is intended to provide results within five minutes and read-out on a standard mobile device.
Matter, Inc. is a biotechnology company based in Atlanta, Georgia. Matter has developed an over-the-counter, at-home, saliva-based SARS-CoV-2 test that runs on the Matter platform which eliminates manual steps and reagents while offering PCR-grade results within 2-minutes. The Matter platform sensitively detects SARS-CoV-2 RNA targets without amplification through novel nanoelectronic and biochemical processes. Clinical trials are ongoing and EUA submission is expected in the near-term.
Pinpoint Science is a diagnostic company based in San Francisco, California. Pinpoint’s technology platform utilizes nanopore sensor technology to combine the speed and simplicity of rapid tests with the sensitivity of lab-based assays. The test is intended to detect SARS-CoV-2 protein antigens within 60 seconds from nasal swabs. EUA submission is expected in the second quarter of 2023.
Senzo is a diagnostic company located in Newtown, Pennsylvania, and London, UK. This funding allows the Senzo team to manufacture and clinically validate their amplified lateral flow rapid antigen test. The test utilizes a novel mechanism of action through the enzymatic amplification of the detection signal on a lateral flow device, providing sensitive results from nasal swabs in less than seven minutes. EUA submission is expected in second quarter of 2023.
Ultimately, partners can pursue appropriate regulatory pathways toward commercialization for their BARDA-funded diagnostic, which eventually may be available to the public through the over-the-counter market.
One of the objectives within BARDA’s 2022-2026 Strategic Plan emphasizes the importance of investing in a portfolio of at-home testing technologies that can empower Americans, improve equitable access, and mitigate the impacts of future pandemics. Additionally, reducing barriers to testing is critical to combating respiratory disease outbreaks, whether due to endemic diseases such as COVID-19, influenza and RSV or a novel respiratory virus with pandemic potential. Adaptable, highly sensitive and specific diagnostic testing technologies are needed to quickly detect the respiratory pathogen, inform earlier treatment, improve equitable diagnostic access, reduce burdens on the health care system, and help mitigate the impacts of outbreaks.
These awards have been made under the Easy Broad Agency Announcement (EZ-BAA) areas of interest AOIs #11a: Home-based, Over-the-Counter Diagnostics for the Detection of SARS CoV-2 and #11b: Enabling Technologies to Support Home-Based Diagnostics for SARS-CoV-2 Acute Infection.
BARDA’s Division of Research, Innovation, and Ventures (DRIVe) established the Easy Broad Agency Announcement (EZ-BAA) to streamline the application process for the review of transformative products and technologies to protect the U.S. from health security threats. Since its inception in 2018, the EZ-BAA solicitation has received hundreds of abstract submissions annually, with awards made in as few as 30 days. The EZ-BAA has helped transform the solicitation process and provides innovators, entrepreneurs, and organizations with a simplified and rapid mechanism to propose ideas to the U.S. government. The application process is both business-friendly and easy to follow.
About 3EO Health:
The following information is provided by the company and does not indicate endorsement by the federal government of the company or its products.
3EO Health Inc. is a “Point of Life” diagnostic company leveraging novel diagnostic technology to power a digital wellness engine. We enable people to thrive by extending the four walls of the health system into the home, work, and community. Our passion is to create a world where every person is empowered to optimize their health.
Wherever life happens, our work:
Enables people to lead wellness for themselves and their families
Improves collaboration between people and their clinicians
Connects people to valuable community resources that underpin health
3EO accomplishes this by creating low-cost ready access to insights via game changing diagnostics and ambient “health supportive” data collection while connecting people with pathways of action to well-being. Our mantra is “…right information, in the right place, at the right time, enabling the right action, right now.” For more information visit www.3EOHealth.com.
About Altratech:
The following information is provided by the company and does not indicate endorsement by the federal government of the company or its products.
Altratech has for the past seven years been researching and developing an alternative to PCR with a focus on non-clinical and home settings. The Altratech cartridge and assay can rapidly detect and quantify molecular targets from any biological sample with no sample preparation or pre-amplification required. The technology brings together nano-technology, semiconductor technology and novel ‘bio-orthogonal’ PNA chemistry to produce a unique and powerful assay. The assay is named Molecular Detection by Proxy and is a magnetic bead-based technology coupled with ultra-sensitive capacitance detection.
About Gladstone Institutes:
The following information is provided by the company and does not indicate endorsement by the federal government of the company or its products.
To ensure our work does the greatest good, Gladstone Institutes focuses on conditions with profound medical, economic, and social impact—unsolved diseases. Gladstone is an independent, nonprofit life science research organization that uses visionary science and technology to overcome disease.
About GRIP Molecular Technologies:
The following information is provided by the company and does not indicate endorsement by the federal government of the company or its products.
GRIP Molecular is a diagnostics company based in St. Paul Minnesota that is redefining medical diagnostics by putting convenient, highly accurate and comprehensive diagnostic testing into the hands of the end user. GRIP’s novel electronic biosensor technology can be used by anyone, anywhere at any time to enable the simultaneous detection of multiple diseases, with one sample and one test, in minutes, using a standard mobile device to facilitate rapid diagnosis and the prompt application of appropriate therapy.
About Matter, Inc.:
The following information is provided by the company and does not indicate endorsement by the federal government of the company or its products.
Matter, Inc., Atlanta, GA, is a health technology company focused on detecting diseases earlier and improving patient outcomes. Matter has developed a next-generation platform technology that eliminates the use of manual steps, reagents, and external equipment while offering PCR-grade results within 2 minutes. This technology decentralizes testing to the home for untrained users. The company has also pioneered scientific approaches that when integrated with the platform, can address unmet diagnostic and prognostic needs across several clinical areas. Matter’s indication pipeline includes SARS-CoV-2, respiratory pathogens, Tuberculosis, Type 1 Diabetes, and maternal health.
About Pinpoint Science:
The following information is provided by the company and does not indicate endorsement by the federal government of the company or its products.
Pinpoint Science is a Bay Area-based startup developing a new generation of bioelectronic point-of-care diagnostics. Our diagnostic platform applies unique nanosensor technology to empower doctors and patients alike with easy-to-use clinical tests that give accurate results in seconds. Specific biomarkers—antibodies, antigens, toxins, nucleic acid sequences—are detected in any biofluid (e.g., saliva, blood, sputum) with an affordable reader, disposable cartridges, and a mobile app. Improving diagnosis speed and accuracy, while dramatically lowering cost, means our products can be deployed effectively in point-of-care settings like physician offices, clinics, pharmacies and at home, as well as at resource-limited regions and for mass screening for pandemic threats.
About Senzo:
The following information is provided by the company and does not indicate endorsement by the federal government of the company or its products.
Senzo is an in vitro diagnostics company developing innovative, accurate, and accessible testing products. Senzo was founded with the vision of utilizing novel technologies, with a focus on enhanced sensitivity, to create mobile, point-of-care and self-testing products and devices with the ability to accurately, quickly, and cost-effectively conduct testing where healthcare professionals and patients need it most. Senzo is creating game-changing products and systems which bring testing to the patient, eliminating the need for the current slow, expensive central-lab testing paradigms. With insights generated at the point of care, patients can make better decisions faster, and healthcare professionals can identify life-threatening diseases at an earlier stage, improving treatment outcomes and saving lives. www.senzo.com.
Link to full article: https://www.medicalcountermeasures.gov/newsroom/2023/athometest/